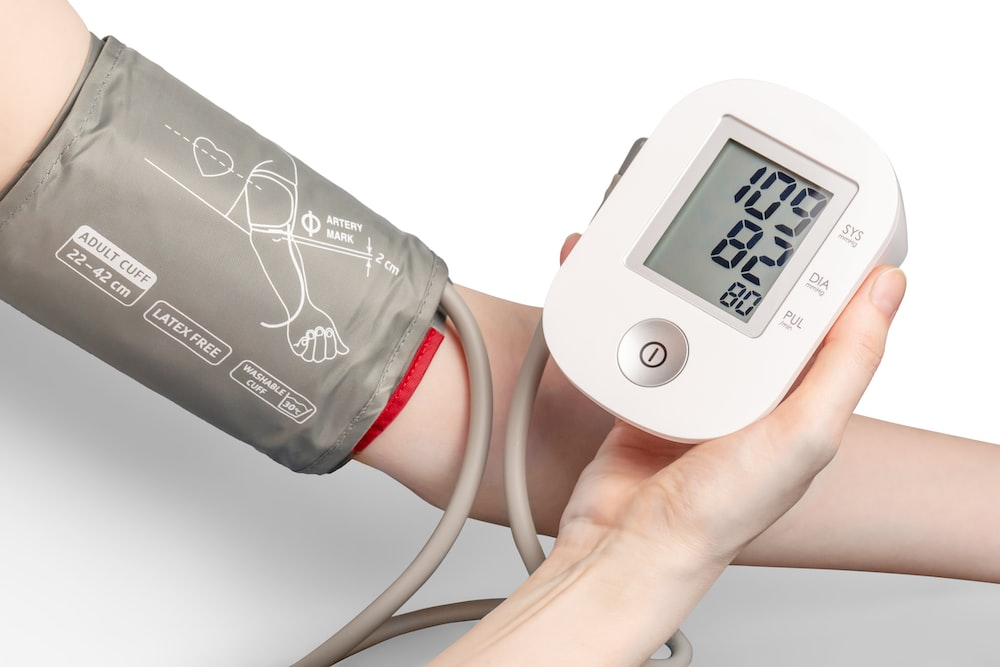
Medical device contract manufacturing is instrumental in the medical field as it offers a cost-effective solution to the production of healthcare devices. While that is the case, it cannot be denied that there are some challenges that this specialized field faces. From regulatory compliance to supply chain management, medical device contract manufacturers face unique obstacles that require careful navigation. In this article, we will explore the 6 main challenges of medical device contract manufacturing, shedding light on the complexities involved in this industry.
Table of Contents
1) Regulatory Compliance
More often than not, product development teams wait until the evaluation process of the devices to start considering issues of compliance. A last-minute approach of this kind usually leads to additional time needed in redesigning the non-compliant devices, hence delaying the awaited product launch. Before contacting the electronics manufacturer, you first need to consider what your target niche would be. The RoHS and REACH, which are the two main sets of regulation, originated in the European Union. Despite that, these regulations are followed almost everywhere in the world, even if unofficially.
2) Supply Chain Management
Managing the supply chain is a significant challenge for medical device contract manufacturers. They must source high-quality raw materials and components from reliable suppliers while maintaining cost-efficiency. Ensuring a stable supply chain, managing inventory, and mitigating the risks of disruptions or delays are essential for meeting production timelines and customer demands.
3) Product Design and Development
Medical device contract manufacturers often face challenges in product design and development. They must collaborate closely with their clients, the original equipment manufacturers (OEMs), to understand their specific requirements and translate them into feasible and compliant designs. This involves expertise in engineering, materials selection, prototyping, and testing to create safe and effective medical devices.
4) Security
Industry 4.0 offers numerous advantages in regards to the future of healthcare – AI, robotics, and cloud computing, among others. This is also a way for contract manufacturers to face liability issues in case of cyber security breach. Currently, there are mandatory tools for reporting that the FDA has put in place to detect issues with devices. Vendors and OEMs should prepare protocols in case security issues occur to allow for development of software or even product recalls.
5) High Production Cost
Regulatory compliance testing as well as approval can be costly, and funding usually increases when manufacturers expand globally. Regulations can differ from one region to another, although various regions of the globe follow the European Union standards or a near-identical spinoff. More often than not, it’s easier and more profitable to come up with an EU-friendly design than create numerous variations for the EU and non-EU nations.
6) Scalability and Flexibility
It’s imperative that device manufacturers are prepared to face fluctuating product demands and the changing dynamics of the market. Scaling up production to meet increased demand or quickly pivoting to new product lines requires careful planning, resource allocation, and operational agility. Balancing scalability and flexibility while maintaining quality standards is crucial for the success of contract manufacturers.
Wrap Up
The above are among the common challenges of medical device contract manufacturing. These challenges call for manufacturers to be proactive so as to streamline the product approval process.